Ceftriaxone Sodium
CEFTRIAXONE SODIUM
Type of Drug:
Semi Synthetic Cephalosporin Derivate
Therapeutic Action and Use:
Broad Spectrum Antibiotic / Antibacterial.
Preparations:
Ceftriaxone Injection
Product Chemistry
Common Name:
Ceftriaxone Sodium
Chemical Name:
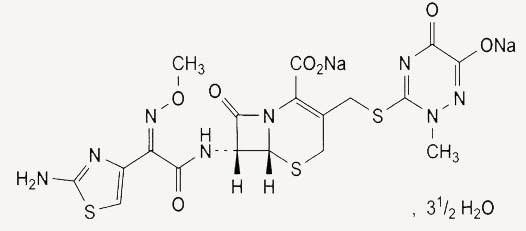
Disodium(6R,7R)-7-[[(Z)-(2-aminothiazol-4-yl)(methoxyimino)acetyl]amino]-3- [[(2-methyl-6-oxido-5-oxo-2,5-dihydro-1,2,4-triazin-3-yl)sulphanyl]methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
Molecular Weight:
662
Molecular Formula:
C18H16N8Na2O7S3,3 1/2 H2O
Chemical Serail No:
(CAS No.) 104376-79-6
Packing:
10Kg in Sterile, air tight, temper proof Aluminium container
Storage:
Store below 25�C. Protect from light.
Shelf Life:
36 months
Related Substances:
i- Individual impurity:
Not more than 1.0%
ii- Total Impurities:
Not more than 4.0%
Sterility:
Meet the requirements
Bacterial Endotoxins:
Not more than 0.08 IU / mg
Contents:
* Ceftriaxone Sodium:
96 to 102 % w/w
Bulk Density(tapped):
Not less than 0.35g/ml
*(Based on anhydrous substances)
Pharmacopeial Quality:
USP, BP, EP
SPECIFICATIONS
Appearance:
A white to slightly yellowish, crystalline powder
Odor:
Characteristic
* Optical Rotation:
-155° to 170°
Water Contents:
8.0 to 11.0 % w/w.
pH Value:
6.0 to 8.0
N,N-Dimethylaniline:
Not more than 20 ppm
